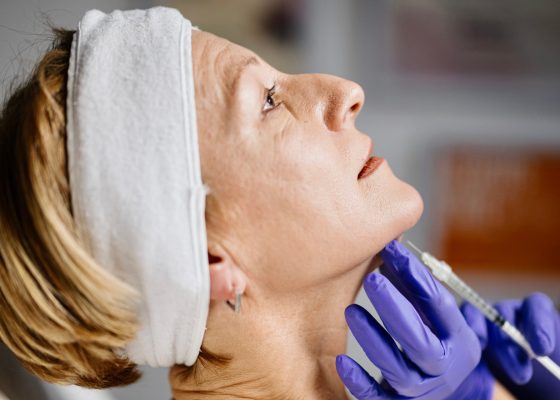
In a major shake-up, nurse-led clinics can no longer purchase stock of Botox or fillers on behalf of doctors or nurse practitioners who do not physically work there.
Hundreds of nurse-led cosmetic clinics in Queensland have now been placed in a potentially unviable situation, after the state health department clarified its interpretation of the Medicines and Poisons Act 2019.
Traditionally, medical cosmetic clinics run completely by registered nurses – i.e. with no nurse practitioner or doctor present onsite – have been able to work around S4 medicines rules which prevent non-prescribers from purchasing and storing prescription-only medicines.
Instead, doctors or nurse practitioners working remotely have been able to order prescription-only cosmetic injectables like botulinum toxin and dermal fillers which are then consigned to nurse-led cosmetic clinics.
When patients present requesting a procedure, they consult a doctor via telehealth, who writes the prescription.
In December 2024, Queensland Health released a fact sheet clarifying that it did not consider these processes legal.
This move caused a considerable amount of panic.
Queensland Health subsequently released a second fact sheet with revisions earlier this month following “engagement with industry”.
Its interpretation of the law did not change, effectively confirming that in the opinion of the Queensland government it is illegal for registered nurses to buy S4 cosmetic injectable medicines, that only prescribers (i.e. doctors and nurse practitioners) can buy S4 cosmetic injectable medicine to hold as stock at a clinic if they physically work there and exercise exclusive custody, and that registered nurses are only allowed to possess S4 cosmetic injectable medicines for the purpose of administering it.
Crucially, this means that nurse-led clinics will no longer be able to purchase stock of injectable medicines in bulk unless they have a prescriber onsite.
Geoff Bloom, a partner at law firm Mills Oakley who specialises in health and life sciences, said Queensland’s interpretation of the law spelled bad news for roughly 600 nurse-led clinics across the state.
“It makes them non-viable,” he told Dermatology Republic.
Mr Bloom argued that, in general, the injectables industry had been operating safely.
“The Medicines and Poisons Act is all about safe handling of medicines and making sure that the right people have them – in that there’s no diversion into the black market – and that they’re properly handled, so that … the cold chain is not broken,” he said.
“This is an industry which has had [very few] instances of unsafe use … so in terms of the policy aims of the legislation, it’s very hard to see why the government would be concerned about what’s happening.
“The effect of what they’re saying is, arguably, to put all of these businesses out of business.”
Poisons legislation is state-by-state, meaning that Queensland’s interpretation of the legislation only applies in Queensland; it is not harmonised like payroll tax.